Clinical Research Center Standard Operating Procedure Center for Clinical and Translational Research Standard Operating Procedur
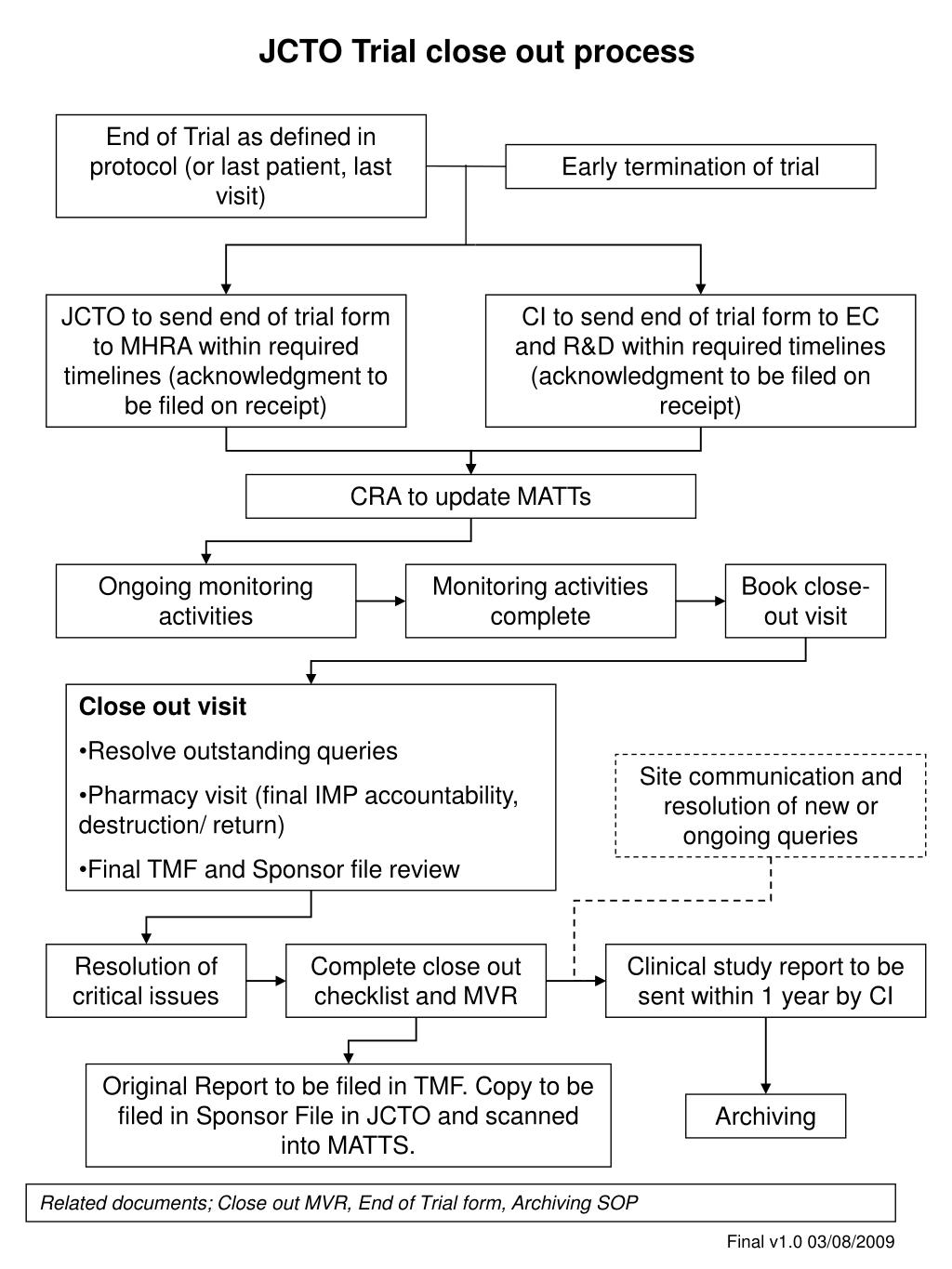
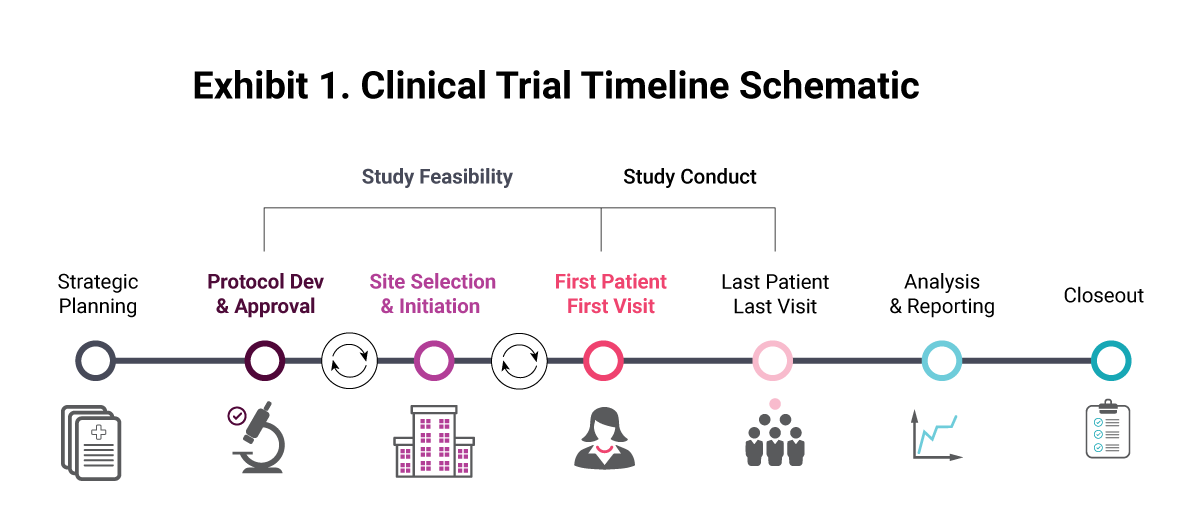
Guidance and Procedure: Closure of Human Subjects Research Studies Overview Closing Sponsor-Initiated Clinical Trials
Page 1 of 5 MONITORING SERVICES FOR A CLINICAL TRIAL EXP_17_2019 Background The Barcelona Institute for Global Health, ISGlobal

Site Closeout Visit Readiness Checklist v20 1375 | PDF | Clinical Trial | Information Technology Management
Study Close Out Visit Beaumont Health 04/04/2018 04/04/2018 Administrative Director Policy Clinical Research SOP's, Research Ins