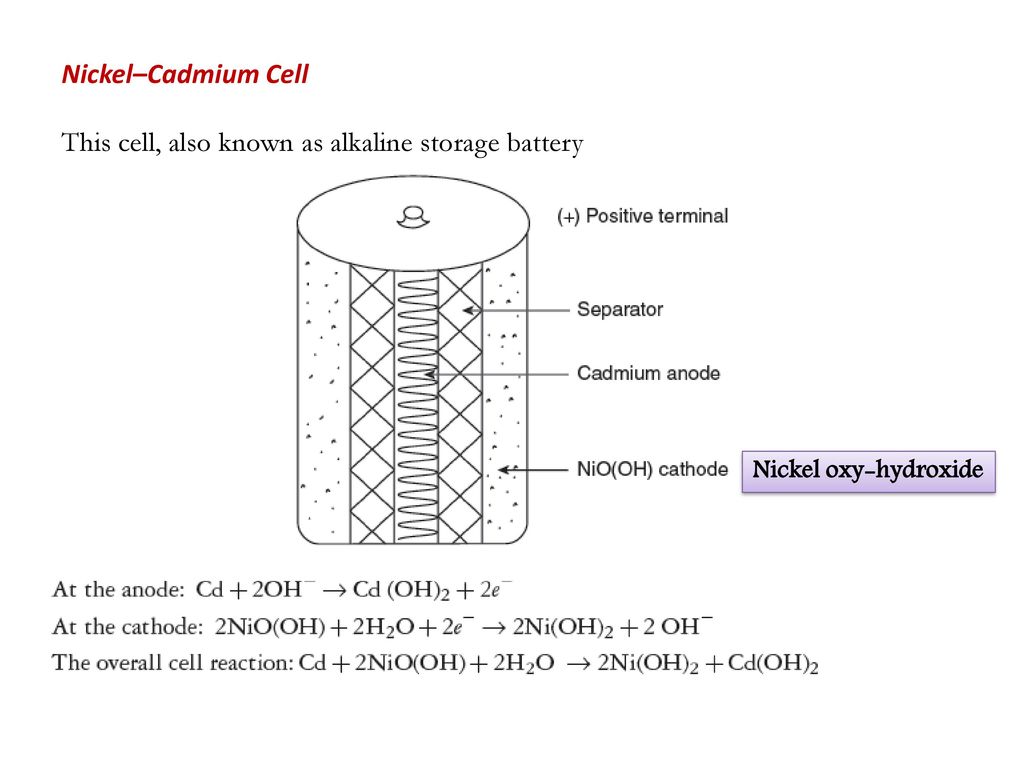
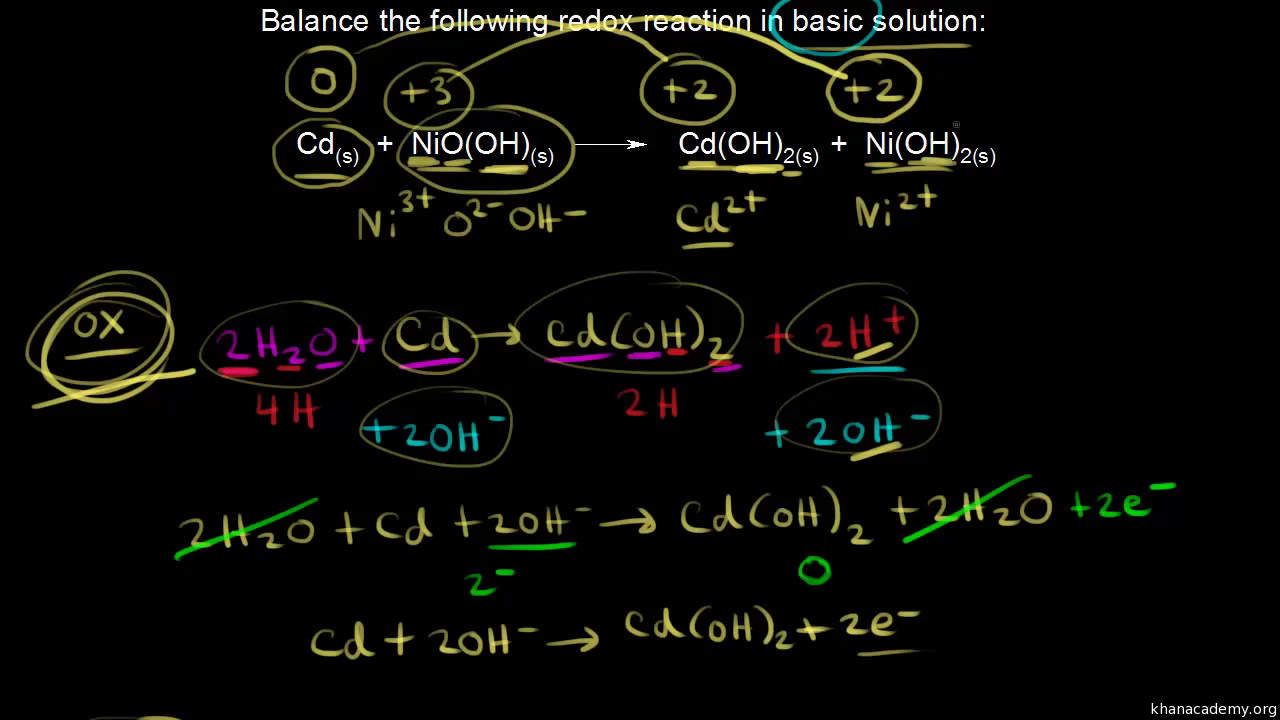
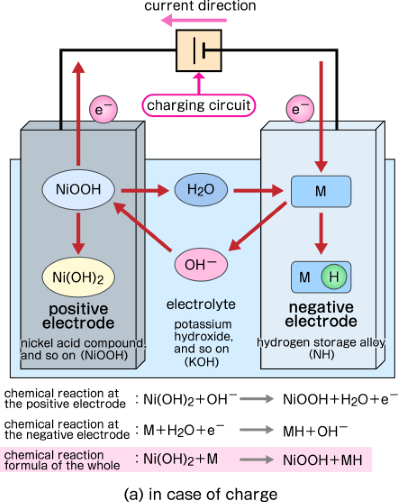
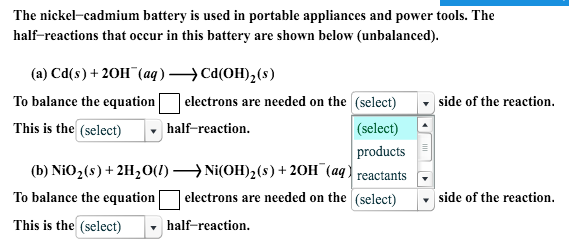
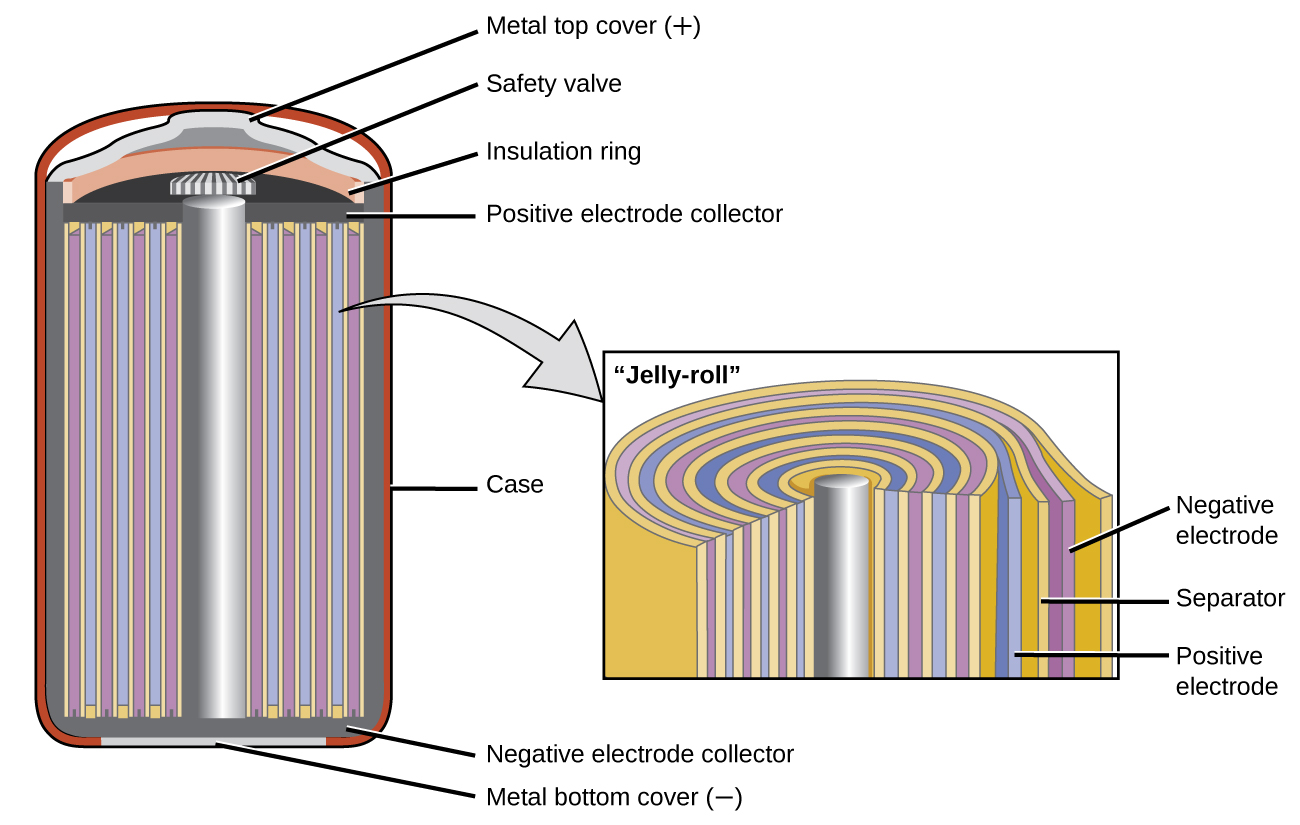
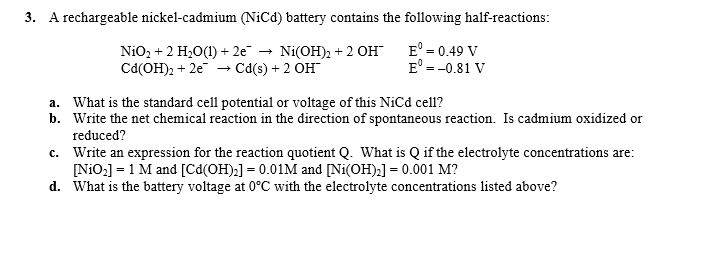
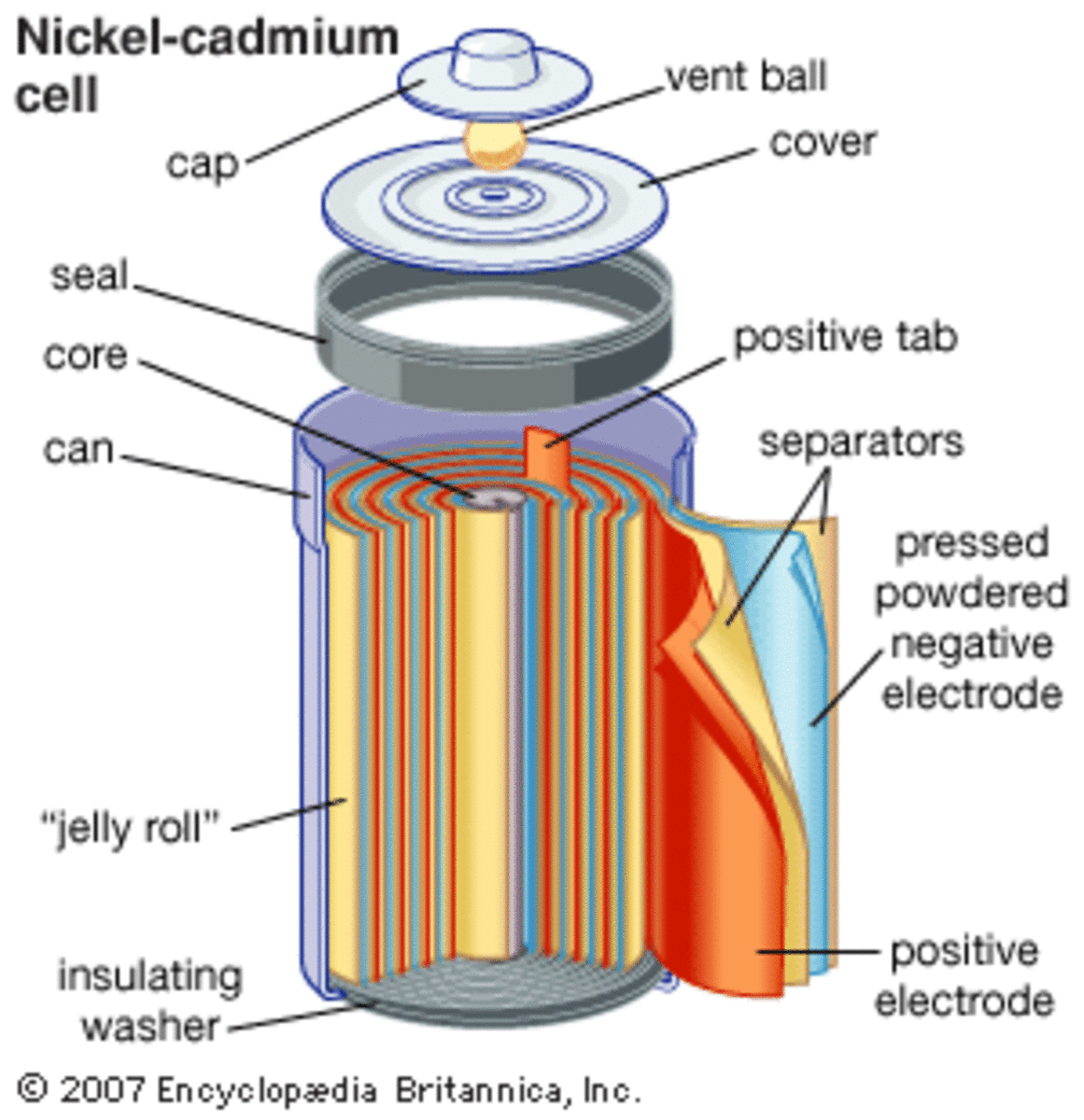
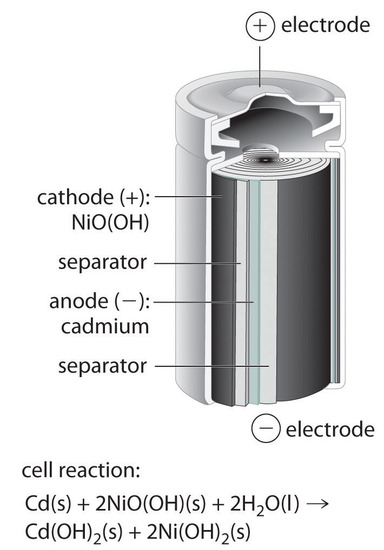
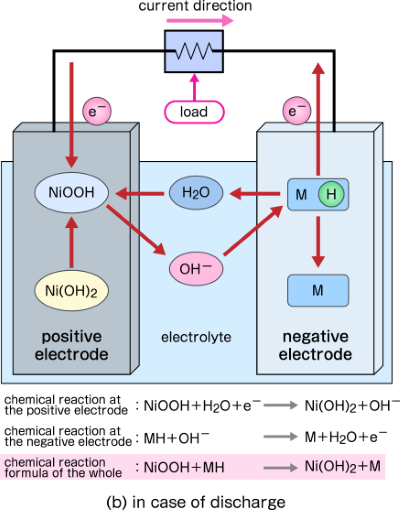
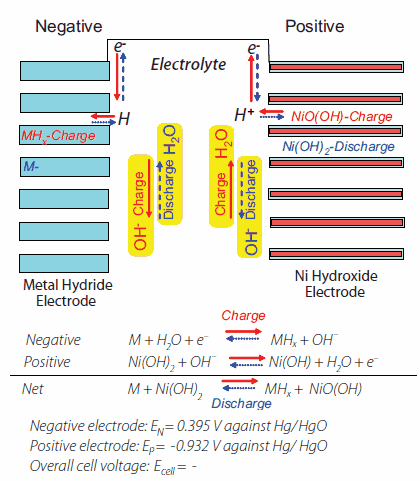
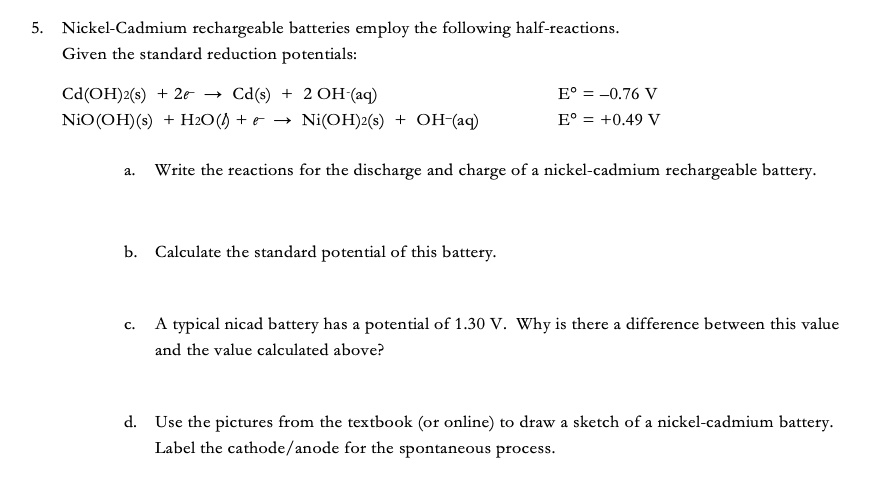
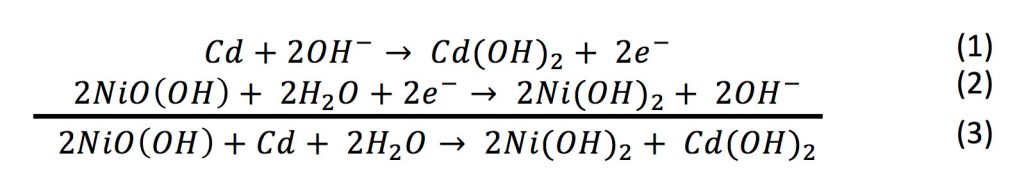SOLVED: Two very common batteries in the ectronic consumer world are lithium-ion batteries and Both of these batteries are rechargeable The nickel-cadmium batteries (NiCd or NiCad) relevant reduction half reactions with standard

What is a Nickel Cadmium Battery? - News about Energy Storage, Batteries, Climate Change and the Environment
![C6 NiCd Rechargeable Battery [HL IB Chemistry] - YouTube C6 NiCd Rechargeable Battery [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/PINXU050LUM/maxresdefault.jpg)